Correction of a Causative Mutation for Hereditary Deafness Restores Hearing with a Single Base Editing Treatment
Correction of a Causative Mutation for Hereditary Deafness Restores Hearing with a Single Base Editing Treatment
- First demonstration of hearing restoration in humanized MPZL2 mutant mice
- Single administration of in-house developed base editor enables tissue repair and ensures safety
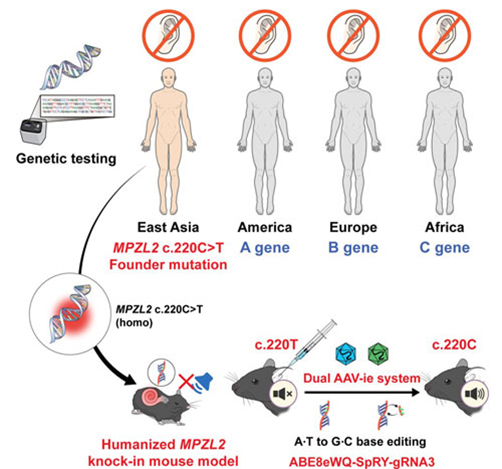
A Korean research team has developed a gene therapy that precisely corrects the representative mutation (c.220C>T) of the MPZL2 gene, which causes hereditary deafness, and has successfully demonstrated hearing restoration with a single injection. This study is the first in the world to validate, at the preclinical level, the therapeutic potential of applying adenine base editor (ABE) precisely to a high-frequency hereditary deafness mutation in East Asian populations, showing the possibility of “one-and-done” gene therapy.
On the 25th, a joint research team consisting of Professor Sang-Yeon Lee (Department of Pediatric Otolaryngology, Seoul National University Hospital) and Professor Sang-Soo Baek (Department of Biochemistry, Seoul National University College of Medicine), together with Sohyang Jung (Neuroscience Program) and Hansol Koo (Cancer Biology Program), announced that they had developed a humanized mouse model carrying the MPZL2 mutation that causes hereditary deafness, and confirmed hearing restoration and auditory cell recovery using their in-house engineered base editor.
The c.220C>T variant of the MPZL2 gene is one of the major causes of DFNB111-type sensorineural hereditary deafness. It occurs with high frequency particularly in East Asian populations and is observed in about 10% of hereditary deafness patients. This nonsense mutation changes a glutamine-encoding CAG codon into a stop codon (TAG), interrupting MPZL2 protein production and leading to rapid hearing loss during adolescence, which gradually progresses to severe deafness. However, no fundamental treatment has been available until now.
The research team analyzed a hereditary deafness cohort of 1,437 families in Korea and abroad, confirmed that the c.220C>T mutation is a major cause, and created a humanized mouse model mimicking this mutation. They then developed a next-generation adenine base editor (ABE8eWQ-SpRY) with improved correction efficiency and accuracy, establishing a strategy to precisely repair the pathogenic base to the normal sequence. This base editor operates by converting adenine (A) to guanine (G) without cutting double-stranded DNA, representing a next-generation gene editing technology with minimal cellular damage and high target specificity.
The research team packaged this base editor into a modified adeno-associated viral vector (AAV-ie) and delivered it through a single injection into the round window of the cochlea. AAV-ie demonstrated high efficiency in delivering to auditory hair cells, and the base editor, guided by sgRNA, precisely reached the mutation site and corrected the pathogenic adenine to the normal guanine.
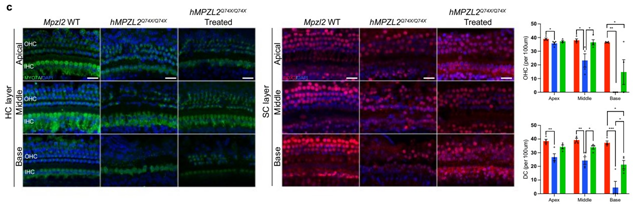
[Figure] Recovery of Auditory Hair Cells and Supporting Cells after Gene Editing Therapy
The therapeutic effect was objectively measured by auditory brainstem response (ABR) and distortion product otoacoustic emission (DPOAE) tests. In the treated group, hearing improvement of about 20–30 dB across all frequency ranges was confirmed, and this effect persisted beyond 20 weeks. This is considered an important result supporting the feasibility and therapeutic efficiency of in vivo gene correction in the inner ear.
Tissue analysis also revealed a significant increase in the survival of auditory cells, such as outer hair cells (OHCs) and supporting cells (DCs), and a marked recovery of cochlear tissue structure. This suggests that gene correction contributes not only to auditory cell function but also to histological restoration.
To evaluate the accuracy and safety of the treatment, the research team used the Cas-OFFinder program at the DNA level to predict possible off-target edits, and verified the absence of off-target effects using GUIDE-seq. Targeted sequencing of cochlear tissue confirmed that no unintended edits occurred outside the target site. At the RNA level, RNA-seq analysis confirmed that no off-target RNA editing occurred, demonstrating that the base editor used in this study possesses both high target specificity and in vivo safety.
Professor Sang-Soo Baek (Department of Biochemistry, Seoul National University College of Medicine) said, “This study is significant in that we precisely targeted a high-frequency hereditary deafness mutation prevalent in East Asia and demonstrated the therapeutic potential of our in-house engineered base editor, thereby providing practical proof of precision gene therapy.”
Professor Sang-Yeon Lee (Department of Pediatric Otolaryngology, Seoul National University Hospital) said, “Until now, treatment for hearing loss has been limited to hearing aids or cochlear implants. In the future, a new path will open for applying personalized gene therapy to patients with clearly defined genetic causes of deafness. We will continue to advance preclinical and clinical research so that this can lead to actual patient treatments.”
Meanwhile, this research was supported by the Seoul National University Physician Scientist Program, the Lee Kun-Hee Pediatric Cancer and Rare Disease Research Project, the National Research Foundation of Korea Young Investigator Program, and the Seoul Bio Project. The results were published in the latest issue of Nature Communications (Impact Factor 15.7), one of the leading international journals.

[From left] Prof. Sang-Yeon Lee (Department of Pediatric Otolaryngology, Seoul National University Hospital), Prof. Sang-Soo Baek (Department of Biochemistry, Seoul National University College of Medicine), Sohyang Jung (Neuroscience Program), Hansol Koo (Cancer Biology Program)