3D Brain Blood Barrier Model Development by SNUH-POSTECH: Leading the Advancement of Neurodegenerative Disease Research
- Reproducing Inflammatory Responses and Permeability Changes of the Brain Blood Barrier with 3D Bioprinting Technology
- Elucidating the Effects of VE-cadherin arrays on Brain Blood Barrier Function and Neuroinflammation
[Figure] Reproducing the blood-brain barrier: Cells derived from a tubular structure created using a brain-vascular-specific extracellular matrix bioink and 3D bioprinting technology self-assemble to form a bilayer structure of the blood-brain barrier.
The research team from Seoul National University Hospital(SNUH) and POSTECH has recently announced the development of a 3D model that accurately replicates ‘the human blood-brain barrier (BBB)’. This model utilizes 3D bioprinting technology to reproduce the blood-brain barrier more precisely than an existing 2D model and is expected to play an important role in the study of neurodegenerative diseases and the development of new treatments.
The blood-brain barrier is a vital protective layer located between the brain and blood vessels. This barrier protects the brain from harmful substances and supplies necessary nutrients and oxygen. However, in neurodegenerative diseases such as Alzheimer's, Parkinson's, and amyotrophic lateral sclerosis (ALS), the blood-brain barrier is damaged or inflamed, worsening the disease. To better understand this issue and develop effective treatment methods, there is a need for a more accurate model of the blood-brain barrier.
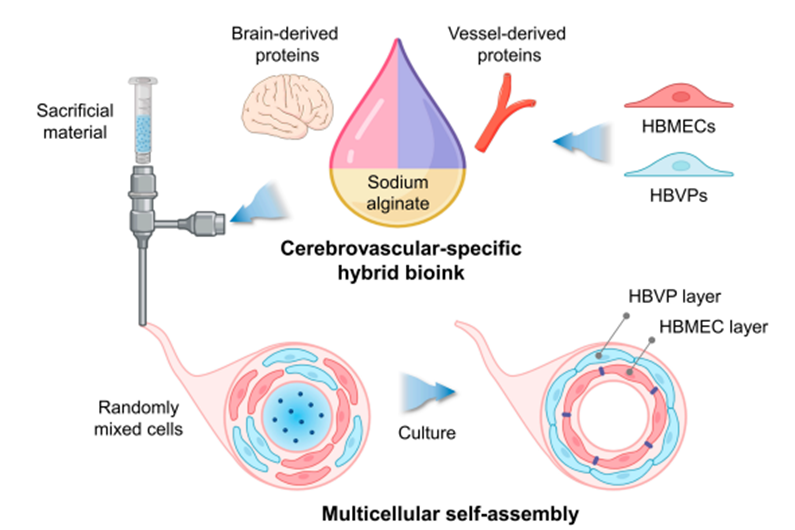
A research team led by Professor Paek Sun Ha from SNUH and Professor Jang Jinah (Han Hohyeon, a PhD student) from POSTECH developed a ‘3D bioink’ that can precisely reproduce the blood-brain barrier using a decellularized extracellular matrix called CBVdECM (Cerebrovascular-Specific Extracellular Matrix). This bioink is an extracellular matrix derived from the brain and blood vessels of a pig and can accurately reproduce the characteristics of the blood-brain barrier.
The research team used this bioink to create a human blood-brain barrier structure using a 3D printer. By fabricating the tubular structure, cells self-assembled to form a double-layer structure and were able to replicate the actual human blood-brain barrier very accurately. In this process, brain microvascular endothelial cells and brain vascular pericytes play an important role, with endothelial cells forming the inner wall of the blood vessel and pericytes surrounding it.
Using the newly developed 3D BBB model, the research team observed changes in the blood-brain barrier when it interacts with inflammatory substances (TNF-α, IL-1β). As a result, they were able to reproduce the key process by which the inflammatory response affects the blood-brain barrier and worsens neurodegenerative diseases. By confirming that inflammatory substances increase the permeability of the BBB resulting in harmful substances penetrating the brain, or the worsening of the inflammatory response, they proved that the blood-brain barrier plays a critical role in neuroinflammation and disease progression.
In addition, the arrangement and organization process of the tight junction protein (VE-cadherin), which was not observed in the existing 2D model, could be clearly reproduced through the 3D model. VE-cadherin is an important protein that helps connect cells and maintains the durability and function of the blood-brain barrier. Through this model, the research team was able to better understand how VE-cadherin regulates the permeability of the blood-brain barrier and provide important information about changes in BBB function in inflammatory diseases.
Professor Paek Sun Ha (Neurosurgery) from SNUH said, “The 3D BBB model developed this time reproduces the blood-brain barrier more precisely and realistically than the existing 2D model and allows us to better understand the role that neuroinflammation plays in neurodegenerative diseases.” He added, “We expect that this will greatly contribute to the development of new treatments and the mechanisms of neurodegenerative diseases.”
Professor Jang Jinah from POSTECH said, “In the future, we plan to develop more precise inflammatory response and BBB permeability quantification technologies by additionally integrating glial cells, neurons, and immune cells, and expand patient-tailored disease models.”
This study was conducted with support from the Ministry of Trade, Industry and Energy, the Korea Planning & Evaluation Institute of Industrial, and the National Research Foundation of Korea. It was published in the international academic journal "Biomaterials Research."

[Pictures from left] Professor Paek Sun Ha from SNUH and Professor Jang Jinah & Han Hohyeon (a PhD student) from POSTECH