Seoul National University Hospital, Seoul National University Bundang Hospital, and Boramae Medical Center: Establishment of CDM Data Integrated Management Platform
- As integrated analysis of 6.85 million people's medical data becomes feasible, innovation in medical big data research is anticipated.
- The cloud-based virtual desktop environment is based on security certification...Outstanding safety and security.
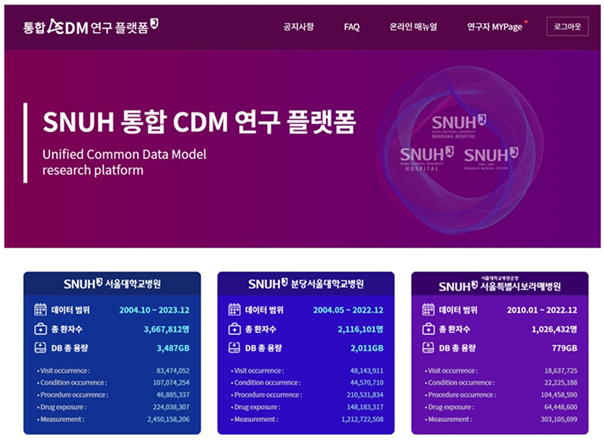
[Picture] Main page of the integrated CDM platform of 3 Seoul National University Hospital institutions
Seoul National University Hospital(SNUH), Seoul National University Bundang Hospital(SNUBH), and SMG-SNU Boramae Medical Center(BMC) have established a collaborative research platform that allows for the integrated data of the three institutions to be viewed and analyzed in one place.
Last month, SNUH(President Kim Young Tae) built an ‘integrated CDM platform’ by integrating the common data models (CDMs) that each institution had managed independently, together with SNUBH and BMC. CDM refers to a data model that standardizes medical data such as medical records, prescriptions, and test results held by each medical institution into a standardized structure.
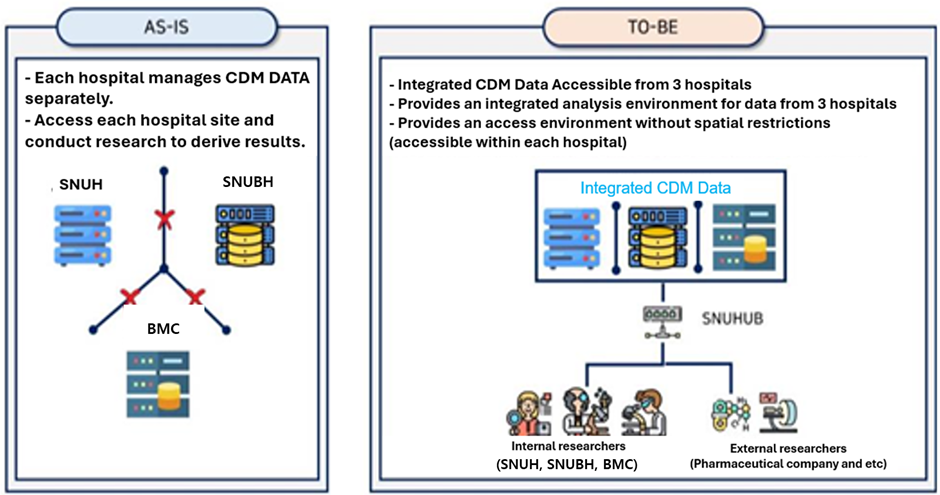
[Figure] Background of the development of the integrated CDM platform
The ‘Integrated CDM Platform’ is a research platform that integrates CDM data from three SNUH institutions, allowing the use of a massive amount of medical data from approximately 6.85 million people. This platform was built over one year, from November 2023 to December 2024, to enhance the value of medical data and foster a collaborative research environment. It builds upon and expands the earlier SNUHUB (SNUH Big Data Platform) previously operated by SNUH.
Previously, the three SNUH institutions had been operating CDM data of the same standard platform. Since each organization managed its data independently, it was difficult for researchers to access data from other organizations, which limited the ability to pursue large-scale analysis or research collaboration.
The integrated CDM platform regularly synchronizes anonymized databases from three institutions, allowing researchers to safely and effectively query and analyze the latest CDM data, such as visit occurrences, diagnoses, procedures, drug exposures, and measurements, in one place. In particular, the platform provides large-scale data that integrates the characteristics of patient groups from different institutions, which can reduce analysis bias and improve research reliability.
This platform operates in a cloud environment that has received CSAP certification (Cloud Security Assurance Program certification) and is accessible only through a virtual desktop environment (VDI) that is completely isolated from the Internet. All data imports and exports require prior approval from the administrator, which helps prevent data leakage and ensures a high level of security.
Researchers can access the platform without any spatial restrictions, as long as they have a web browser. They can also conduct necessary analyses using basic research software, which provides excellent convenience. This service is available not only to researchers at the three institutions but also to external researchers, such as those from pharmaceutical companies and other organizations engaged in joint research. This initiative is expected to promote the expansion of the domestic bio-health research network.
SNUH Group, which has established a short-term data-based joint research environment through the integrated CDM platform, plans to gradually advance the data integration system in the future to complete the data pipeline and integrated analysis platform that collect medical big data (clinical and genomic data, lifelog, etc.).
SNUH President Kim Young Tae said, “The construction of this integrated CDM platform is the first step of cooperation to implement the ONE SNUH Network and is expected to become the cornerstone of data integration and sharing for the advancement of domestic medical care.” He added, “SNUH Group will continue research for patient treatment innovation and do its best to strengthen the global competitiveness of medical research in Korea.”