Seoul National University Hospital to expand CAR-T treatment for patients in the blind spot of Kymriah insurance
- Kymriah, insurance coverage only for relapsed/refractory patients whose bone marrow accounts for more than 5% of leukemia cells...
- Request to expand the number of clinical research patients to aid treatment of patients with acute lymphoblastic leukemia not covered by insurance, approved by the Ministry of Health and Welfare
On October 13th, Seoul National University Hospital announced its proposition after requesting the Ministry of Health and Welfare to expand the number of patients in CAR-T clinical studies in order to expand treatment support for patients who are not covered by insurance for Kymriah, a CAR-T treatment, passed regulations procedures. If approved by the Ministry of Food and Drug Safety, it is expected that additional CAR-T treatment will be provided to patients in the blind spot of Kymriah insurance.
CAR-T treatment is a customized treatment in which immune cells (T cells) obtained from a patient's blood are genetically engineered to recognize cancer, then cultured and put back into the patient's body. It is attracting attention as an innovative and up-to-date treatment because immune cells accurately target only cancer cells while minimizing damage to normal cells in the body.
Currently at SNUH’s Department of Pediatrics and Adolescent care, Professor Kang Hyung-Jin's team, through the Ministry of Health and Welfare's advanced regenerative medicine clinical research support project, are conducting a researcher-led clinical study on hospital-produced CAR-T for relapsed and refractory children and adolescents and young adults under the age of 25 with acute lymphoblastic leukemia. This clinical study was prepared before the introduction of Kymriah, a CAR-T treatment, and was scheduled to end when Kymriah was covered by insurance.
However, Kymriah insurance was applied only to relapsed or refractory patients whose leukemia cells account for more than 5% of their bone marrow. As a result, it has become difficult for patients with micro leukemia (leukemia cells account for less than 5% of the bone marrow) or extramedullary recurrence to access treatment.
The reason why Kymriah insurance is applied only to patients with bone marrow recurrence in Korea is that domestic insurance approval clinical trial standards are based on evidence. During the development of Kymriah, patients with more than 5% of leukemia cells in the bone marrow were registered to confirm the effect during its clinical trial, and insurance standards in Korea were determined based on this data.
Accordingly, SNUH, which has been treating leukemia patients by preparing the entire process from production of CAR-T treatment to patient treatment after administration, has stepped forward for the first time among domestic hospitals since April. Among the patients being treated at SNUH, there were also patients with extramedullary recurrence, and the leukemia mass disappeared.
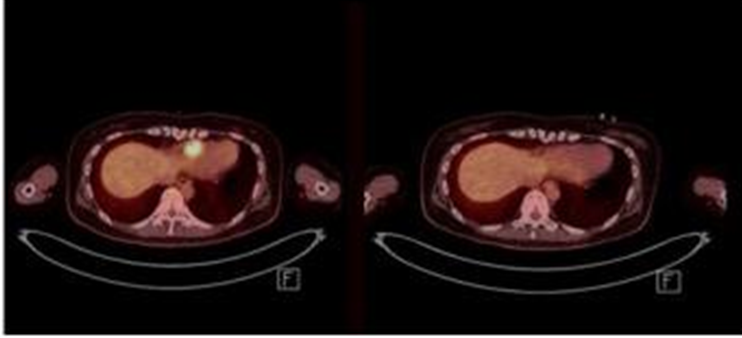
[Image] The leukemia mass next to the heart completely disappeared with Seoul National University Hospital's self-produced CAR-T treatment. (Mass: bright yellow; before treatment on the left side, after treatment on the right side)
Based on these treatment results, we requested the Ministry of Health and Welfare to expand the number of patients in clinical research to provide treatment opportunities to patients with micro leukemia and extramedullary relapses under the age of 25 who need CAR-T treatment but are not covered by insurance – the proposal was passed in a recent review.
SNUH plans to provide additional opportunities for CAR-T treatment to leukemia patients who are in the blind spot of Kymriah insurance after passing the Ministry of Health and Welfare review for the expansion of subjects for this CAR-T clinical study.
Professor Kang Hyeong-Jin, who is in charge of the research, said, “We expect that patients in the blind spot of insurance will be covered by insurance in the near future, but we applied for additional patients this time for patients who need CAR-T treatment even before that.”
“Kymriah was developed at a university and was transferred after showing successful results in an early researcher-led clinical trial. Unfortunately, however, Korea does not have a virtuous cycle system in which technologies developed at universities, hospitals, and research institutes go through researcher-led initial clinical trials and then are transferred to companies.” He emphasized, "I hope that researcher-led clinical trials will be initiated in Korea based on the 'CAR-T production, administration, and treatment management integrated system' established by Seoul National University Hospital."

[Photo] Department of Pediatrics, Professor Kang Hyung-Jin