A Way to Fast Cure is Found: Chronic Hepatitis B, a Disease of Life-long Treatment
- Vaccination given after injecting antiviral agent + pegylated interferon
- Hepatitis B surface antigen (HBsAg) serum was cleared from 16.2% of 111 patients studied
The antiviral agent used by most patients of chronic hepatitis B throughout their lifetime can control the hepatitis B virus, but cannot clear it. However, a recent study shows that if vaccination is given after injecting the antiviral agent and pegylated interferon, that there will be a high chance of curing the disease within a short period of time.
On December 10th, the research team of Professor Kim Yoon-Jun and Professor Lee Jeong-Hoon in the Department of Internal Medicine at Seoul National University Hospital presented their research results about giving vaccination to 111 chronic hepatitis B patients in whom the virus had been controlled by entecavir, an oral antiviral agent, after a combined treatment with pegylated interferon.
The research team divided the patients into groups of 37 each, as follows: ▲ group A, vaccinated after 1 month of entecavir + pegylated interferon treatment ▲ group B, vaccinated concomitantly with entecavir + pegylated interferon treatment ▲ group C, entecavir injected, only. Results were checked after 100 weeks.
The goal of treating chronic hepatitis B is the clearance of hepatitis B surface antigen (HBsAg) serum, considered as the proof of functional cure. Patients who have been cleared of HBsAg serum are at low risk for cardiac cirrhosis or liver cancer.
According to the results, the group vaccinated after 1 month of drug treatment showed a significantly high level of HBsAg serum clearance. Clearance rate was 16.2% with 6 out of 37 patients cleared of HBsAg serum. This means that the virus was cleared in 1 every 6 patients.
<Chronic Hepatitis B Treatment Groups>
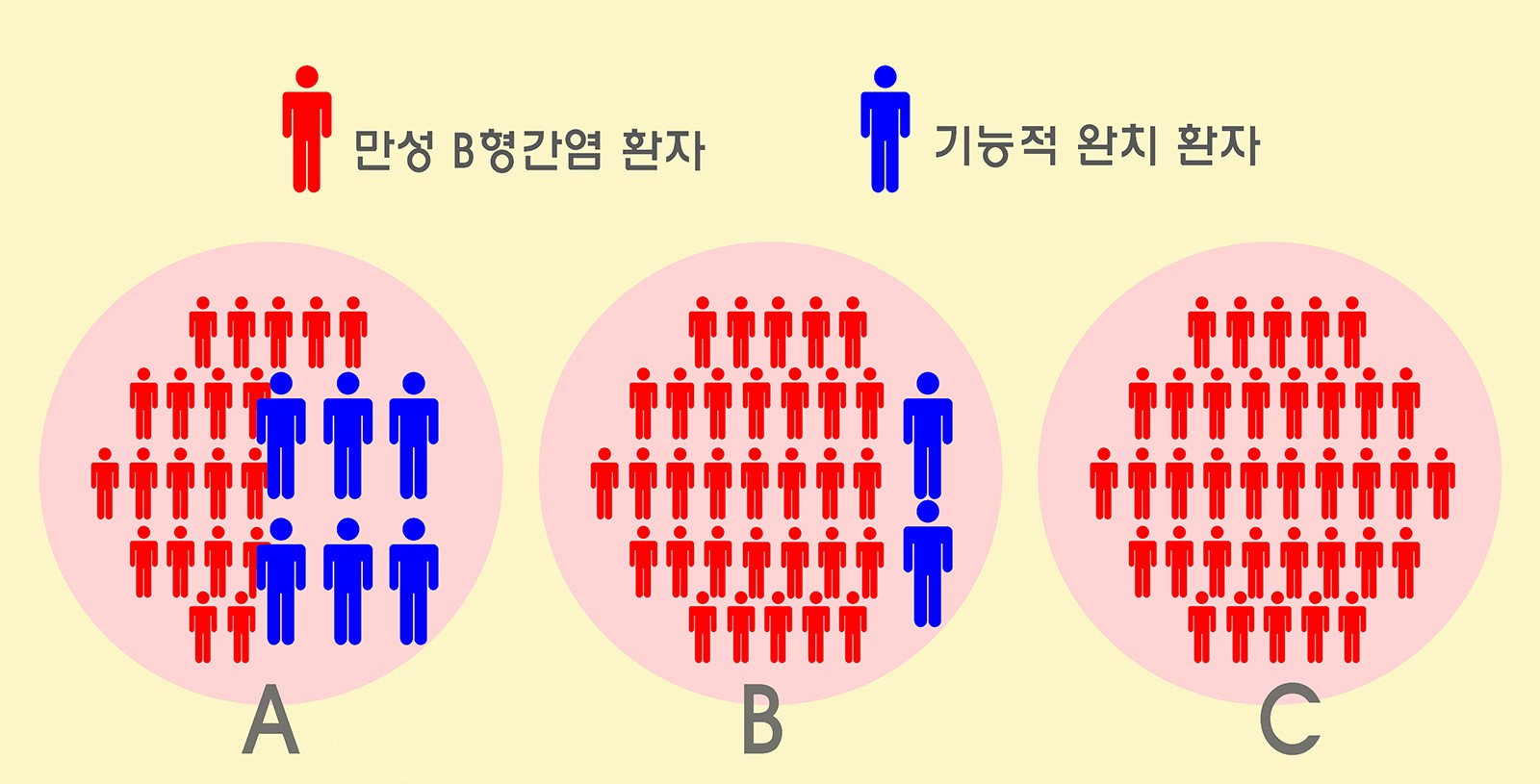
A: Group vaccinated after entecavir + pegylated interferon treatment. After 100 weeks, 6 patients were functionally cured, cleared of HBsAg serum.
B: 2 patients were cured in the group vaccinated during the entecavir + pegylated interferon treatment.
C: No patient was cured in the group that received only the entecavir injection.
Chronic hepatitis B, settled all throughout the world, induces cardiac cirrhosis and liver cancer. Presently, we can prevent these diseases by using an oral antiviral agent, which can effectively control the virus. However, the rate of patients being cleared of HBsAg serum is only 0.8% per year. This means that it takes 52 years for a patient to be cleared of HBsAg, and thus, patients are forced to take drug through their lifetime.
Former studies have showed that when pegylated interferon is used concomitantly with the oral antiviral agent, the rate of HBsAg clearance becomes much higher than when the oral antiviral agent is used alone. However, because of its side effects and low cost-efficiency, it has failed to be considered as standard treatment.
The new study by the research team at Seoul National University Hospital is the first study in the world that which has shown that the rate of HBsAg serum clearance can be improved by adding vaccination to the treatment of using both the oral antiviral agent and entecavir.
It should be noted that a high cure rate of 16.2% was achieved in the new study merely by combining 3 types of widely-used drugs, instead of a new drug. Patients can now stop taking the oral antiviral agent they were required to take throughout their lifetime.
“With the oral antiviral agent treatment alone, it takes patients a lifetime to clear the HBsAg serum, but with our new treatment strategy, chronic hepatitis B patients can now look forward to being functionally cured within 2 years of treatment,” explained Professor Kim Yoon-Jun.
The research team members expect that, with their study results, the treatment period of chronic hepatitis B will be shortened, and that patient lives will be improved, while they also mentioned the need for more study cases on a larger scale.
“We were able to come up with good results by doing a randomized controlled proof-of-concept study with available drugs,” said Professor Lee Jeong-Hoon, the first author of the study. He predicted that “accumulation of more study cases shall open the path to curing chronic hepatitis B in a short period of time.”
The research team’s study was published in the online version of a recent volume in the international journal, Clinical Infectious Disease.