Percutaneous Human Application of New Generation Artificial Bioprosthetic Heart Valves in the Pulmonary Valve Position Without Immune Rejection
Percutaneous Human Application of New Generation Artificial Bioprosthetic Heart Valves in the Pulmonary Valve Position Without Immune Rejection
- Seoul National University Hospital research team developed new-generation artificial bioprosthetic heart valve, which is being on the clinical trial.
- New-generation valve was transplanted successfully by simple percutaneous interventional treatment
- Enhanced quality of life to patients of valvular heart disease, Korea‘s artificial valve export expected
 Seoul National University Hospital research team developed world’s first artificial bioprosthetic heart valve that minimizes immune rejection, and succeeded in applying to a patient. The team also was successful in transplanting the valve with a catheter intervention, much simpler than a open heart surgery.
Seoul National University Hospital research team developed world’s first artificial bioprosthetic heart valve that minimizes immune rejection, and succeeded in applying to a patient. The team also was successful in transplanting the valve with a catheter intervention, much simpler than a open heart surgery.
These valves secures better hemodynamic profiles and durability than the existing bioprosthetic heart valves, thereby greatly reducing reoperation from limited artificial bioprosthetic heart valve durability.
Seoul National University Hospital research team led by Professors Yong Jin Kim/Hong Gook Lim of thoracic surgery and professor Gi Beom Kim of pediatrics developed a ’new-generation artificial bioprosthetic heart valve (“new-generation valve”) that minimizes immune rejections, a common problem in xenogrft, by using a pig‘s pericardium and giving special immune and chemical fixing treatments. The valves maintained normal shapes and functions for over 6 months of observation period, after transcatheter implaning these valves to sheep in 2011. This research results were introduced to an internationally renowned journal of ‘International Journal of Cardiology’ in 2014.
The research team received approval from the Korean FDA to conduct a new-generation valve implantation on pulmonary area to a patient (22, female) who received surgery several times due to congenital heart disease.
When the right ventricle pumps blood to the lung, the pulmonary valves prevent the bloods from refluxing to the right ventricle. The patient was diagnosed with tetralogy of Fallot at a young age, and received surgery to widen the narrow area of pulmonary valve and artery. The patient did not have pulmonary valve function later that resulted in blood reflux in right ventricle and low performance. After the new-generation valve was implanted by transcatheter technique, blood reflux disappeared and the patient discharged healthy without any complication.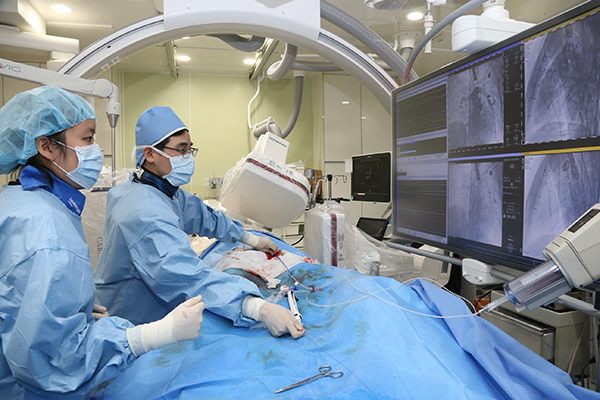 The research team, using ‘Nitinol stent’ developed with Taewoong Medical Co., Ltd., implanted new-generation tissue valves to the right ventricular outflow tract.
The research team, using ‘Nitinol stent’ developed with Taewoong Medical Co., Ltd., implanted new-generation tissue valves to the right ventricular outflow tract.
Recently artificial bioprosthetic heart valve implantation, rather than open heart surgeries, a new implantation method has been popular, whereby stented valves are inserted in groin vessels by transcatheter technique.
Currently, stented-artificial bioprostheic valves were developed mainly in US and European countries, are commercialized and being implanted to aortic valve stenosis especially in elderly patients. The stented artificial bioprosthetic valve developed by the research team is specialized for diseased heart valve and has a difference in immune reaction. Especially, trascatheter implantation of stented artificial bioprosthetic valve in pulmonary position is first time in Korea.
Professor Gi Beom Kim said, “Stent-artificial valves specialized for pulmonary valve diseases are still under development. The fact that we have developed a valve minimizing immune rejections and actually conducted a development with domestic technology, bears a significant meaning. If this transcatheter implantation of stented artificial bioprosthetic valve is commercialized, patients suffering from pulmonary valve disease can be treated with simple interventional technique and the longer durable immunologically modified bioprosthetic valve with reduced reoperation rates.”
The research team, to develop the new-generation stented tissue valves, continued their research work with Bio- xenotransplantation (supported by the Ministry of health and welfare services, hosted by Seoul National University Hospital) for almost 10 years. All the source technology and patent related to the research results are transferred to Taewoong Medical, a Korean company. To localize the valves, the team has shaken off the love call from the world‘s largest valve company.
Professor Yong jin Kim (Bio-xenotransplantation , Development of cardiovascular xenotransplantation, head researcher) stated, “This new-generation heart valves will change the artificial bioprosthetic valve market dominated by developed countries and become an important event to open a new generation of Korean medical technology.”
The research team will additionally implant this new-generation heart valves (pulmonary valves) to other patients, and prepare to commercialize. The application area of the valve will expand from pulmonary valve to aortic valve and others.